| University | Nanyang Technological University (NTU) |
| Subject | CH2123: Chemical Thermodynamics |
Instructions:
- This is an individual assignment. Any form of plagiarism will be penalized according to NTU’s academic integrity policy.
- Answer all 3 (three) questions. If necessary, you may need to find additional information from external resources and provide the reference you use.
- Submit your answers to Turnitin on NTULearn by 21 April 2025 at 17:00. Please only do a single file submission. Late submission will not be accepted; therefore please submit early to avoid any technical issue.
- The solutions can be handwritten (make sure it is legible) or typed. You can submit the soft copy, scanned or pictures of the solutions. Please ensure high resolution of the picture before submission.
- Please combine all of your workings, including Excel file, into one ZIP file and submit it to Turnitin.
- Homework will be graded on efforts, not the final answer obtained. However, give your best effort in solving the problems to enforce the concepts you have learned. Below is the detailed rubric for the continuous assessment, which the homework is a part of.
Hire a Professional Essay & Assignment Writer for completing your Academic Assessments
Native Singapore Writers Team
- 100% Plagiarism-Free Essay
- Highest Satisfaction Rate
- Free Revision
- On-Time Delivery
Question 1 (Fugacity of real gas)
By using Lee/Kesler generalized correlation below, calculate the fugacity of isobutane at 154 °C and 8620 kPa.
Generalized correlation defines the compressibility factor of a pure component as:
Z = Z₀ + ωZ₁
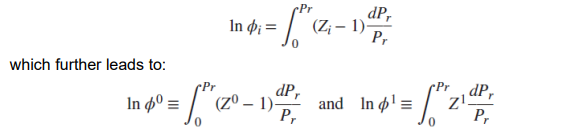
where Z₀ and Z₁ are functions of (Pr, Tr), which are the reduced pressure and reduced temperature, respectively, and ω is the accentricity factor of the pure component.
The equation for fugacity coefficient φi can now be written in terms of reduced pressure, which further leads to:
(Refer to Appendix D Tables D.13-D16, Smith Van Ness, Introduction to Chemical Engineering Thermodynamics 8th Ed., 2018)
Question 2 (Vapor-Liquid equilibrium model)
The vapor-liquid equilibrium data for a binary liquid solution containing acetone (1) – methanol (2) at 55 °C is given in Table 1. Assume that the vapor phase can be considered as ideal gas mixture whereas the activity coefficients for acetone (1) and methanol (2) can be expressed by Van Laar model as follows:
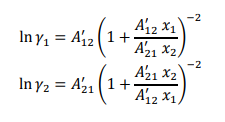
Buy Custom Answer of This Assessment & Raise Your Grades
- Determine the parameters A’₁₂ and A’₂₁ (both of which are constants) that best fit the data in Table 1.
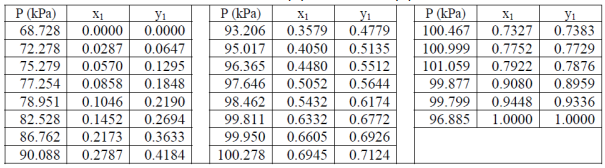
- By using Excel, construct P-x, y diagram at 55 °C based on the suggested model above, and compare it with the experimental data (see Table 1).
- Compare the azeotropic composition based on the experiment and the model above.
Note: The saturated vapor pressures of each component can be determined by using Antoine equations from any textbook/other reference.
Table 1. VLE data for acetone (1) – methanol (2) mixture at 55 °C.
Question 3 (Henry’s law application)
Aqueous solution of perfluorotributylamine can be considered as artificial blood because of its high oxygen solubility.
At 25 °C and an oxygen pressure of 1 atm, 380 mL of oxygen gas (measured at 25 °C and 1 atm) dissolves in 1 liter of pure perfluorotributylamine, which has a liquid density of 1.88 g/mL.
- Determine the Henry’s Law constant, in unit of atm, for oxygen dissolved in pure perfluorotributylamine.
- Suppose a solution contains 10 vol% perfluorotributylamine and 90 vol% water. Estimate the volume of oxygen gas (measured at 25 °C and 1 atm) dissolved in a liter of the solution when it is equilibrated with air at 25 °C. Note that the Henry’s constant for oxygen in water at 25 °C is 4.34 × 10⁴ atm.
Note: The physical properties of perfluorotributylamine can be obtained from external reference.
Stuck with a lot of homework assignments and feeling stressed ? Take professional academic assistance & Get 100% Plagiarism free papers
Is your CH2123 Fugacity, VLE Modeling & Henry’s Law assignment not going to be done due to lack of time? No worries! You are at the right place. Our platform provides the best Singapore Assignment Help. We have knowledgeable and skilled writers who can give you high-quality, plagiarism-free solutions and assignments with original content. And we help you to stand out from the rest, along with getting high grades. We also provide free sample assignments to help you. Contact us right away.
Looking for Plagiarism free Answers for your college/ university Assignments.
- BAFI1045 Assignment -Constructing and Evaluating Passive and Active Portfolios Based on the Straits Times Index (STI)
- PSB501EN Assignment 1: Engineering Systems Integration: A Multi-Technique Approach to Mechanical Analysis
- FIN2210E/FIN2212E Group Assignment: Financial Risk Management Analysis of Bursa Malaysia Companies
- FLM101 Assignment: A Cinematic Dissection: Stylistic Elements and Their Thematic Significance
- Assignment: Transforming Talent in the AI Era: From War to Wealth through Ecosystem Innovation
- COMP 1105 Assignment: Health-Focused E-Commerce Website: A Web Technologies Project Using HTML5, CSS, and JavaScript
- Assignment: Machine Learning in Robo-Advisory Services: Evolution, Applications, and Future Trends
- OMGT2229 Assignment: Quantitative Forecasting, Economic Order Analysis, and Strategic Sourcing Decision-Making for JB Hi-Fi
- Assignment 2: Corporate Finance and Planning: An In-Depth Financial Analysis of Company
- BUSM4551 Innovation Management Assignment: Innovation and Its Role in Advancing the UN SDGs

